Residual Solvents in Packaging Film
Introduction
Volatile organic compounds can be released from packaging materials. The determination of these compounds can be useful in assisting in the development and manufacture of packaging materials having minimal retained packaging solvents.
This work deals with determination of the amount of residual solvents released from a packaging material contained in a sealed vial under a given set of time and temperature conditions.
The packaging samples are analyzed by headspace-GC-FID. Each sample is analyzed with a GC equipped with an HT200H autosampler.
Experimental conditions
First of all, a preliminary test is performed to check the system reproducibility.
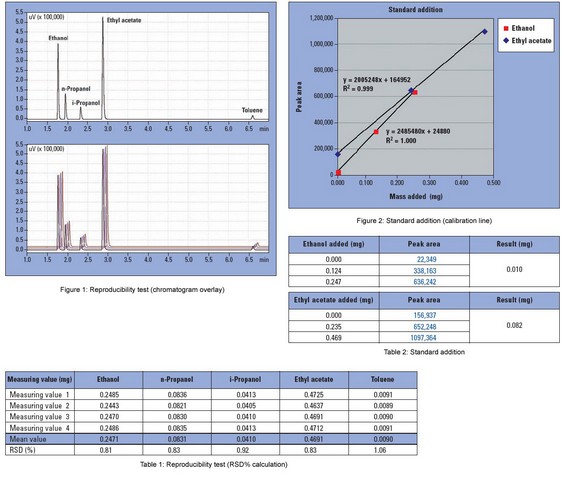
1uL of a solvent mixture (Ethanol, n-Propanol, i-Propanol, Ethyl acetate, Toluene) is added to four empty headspace vials.
Due to the low solvent amounts, the solvents totally evaporate during incubation. This process is called “total evaporation”. No matrix is present in the vials and so there is no alteration to the solvent concentrations in the gas-phase due to interfering substances. The solvent quantification can be performed using external or internal standards.
The results of the solvent residue analyses are shown in Table 1.The values reported are the absolute masses in the headspace vials. The outstanding reproducibility of the analysis is shown in the chromatograms in Figure 1.
After the preliminary test, the packaging foils analysis is performed.
The packaging foils analyzed in this work contain low amounts of ethanol and ethylacetate solvent residues. For the quantification, foil samples of specific surface areas (50 cm²) were prepared by insertion into headspace vials. The samples are heated to a specific temperature for 15 minutes. When equilibrium is reached, a volume is drawn from the gas phase(headspace) of the vial and injected into the GC and quantified.
The presence of the foil provokes a matrix effect, the consequence is that the solvent residues do not evaporate completely into the gas-phase. In detail, a state of equilibrium is established between the gas-phase and the foil constituents. This matrix effect must be considered during quantification. A calibration in the presence of the matrix – in this case the foil – is therefore recommended. Consequently, a calibration is performed in the form of a standard addition of the solvent mixture in Table 1. 0.5 uL and 1.0 uL of the solvent mixture are added to two identical samples (foils in headspace vials) and subsequently analyzed. The solvent residue concentration in the foil can be calculated from the slope and intercept of the calibration line shown in Figure 2. The quantification results are shown in Table 2.
When the peak areas obtained from standard addition analyses are compared with the data obtained from complete evaporation, significant deviations are clear. Using standard addition, the peak areas are up to 30% smaller. This is due to the matrix effect. The matrix effect magnitude depends on the analyzed sample and the type of matrix.



