Solventi Residui in Prodotti Farmaceutici
Reference Method: USP 467
Introduction
Residual solvents in drugs are organic volatile that are used or produced in the manufacturing of active substances or excipients, or in the preparation of medicinal products. Because these chemical residues may be hazardous to human health and to the environment, the drug manufacturer must ensure that they are either not present in their products or are present only below acceptable levels.
The International Conference on Harmonization (ICH) publishes a guideline (Q3C) listing the acceptable quantity of residual solvents that can be present. In this guideline, residual solvents are divided in classes, according to their toxicity and to the degree of environmental hazard.
The three main categories of solvents are:
- Class 1: Class 1 solvents are known human carcinogens that pose a risk to both the consumer and the environment. They should be avoided in the production of drug substances, excipients, or drug products unless their use can be strongly justified in a risk-benefit assessment.
- Class 2: Class 2 solvents are nongenotoxic animal carcinogens, characterized by less severe toxicity. Solvents of this class should be limited in pharmaceutical products in order to protect patients from potential adverse effects.
- Class 3: Class 3 solvents have low toxic potential to humans.
The United States Pharmacopeia (USP) general chapter <467> has provided a method for the determination of Class 1 and 2 residual solvents.
In detail, the USP <467> method consists of a static headspace extraction coupled with a gas chromatographic separation and flame ionization.
The method consists of three procedures (A, B, and C), that are designed respectively to identify, confirm, and then quantify residual solvents in drug substances and products.
Procedure A is the first step in the identification process and is performed with a G43 (6% cyanopropylphenyl-94% dimethyl polysiloxane) column. It is used to determine if any residual solvents is present in the sample at detectable levels.
If a compound is determined to be above its specific concentration limit, then Procedure.
B is used to confirm this result. A G16 (polyethylene glycol) capillary column is used in procedure B because it has an orthogonal selectivity compared to the G43 column. So co-elutions that occur with one phase will be resolved with the other phase.
Once a residual solvent has been identified and verified, Procedure C is used to quantify the analyte. G43 or G16 columns can be used, depending on which has provided the most definitive results (lower analyte co-elution). Each procedure involves system suitability criteria followed by sample analysis.
Experimental conditions
In this work the procedures are performed with a GC equipped with an HT200H autosampler.
This work is designed to demonstrate that all products and instruments used permit to respect the system suitability criteria and so can be used for the residual solvents in drug analysis.
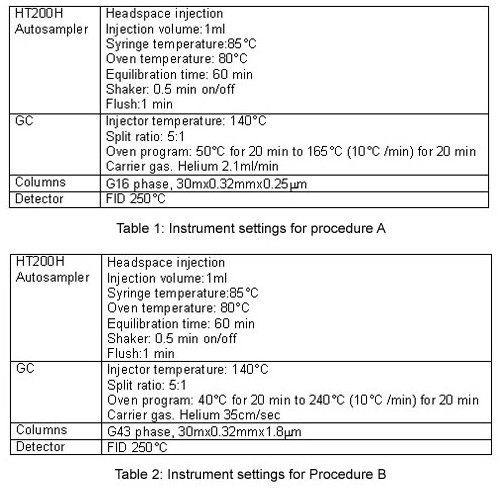
Procedure A
The system suitability requires running 3 solutions, the Class 1 system suitability, Class 1 standard, and the Class 2 mixture A standard solutions.
The criteria which must be met are:
- For the Class 1 system suitability solution, each peak must have a S/N ratio not less than 3.
- For the Class 1 standard solution, the 1,1,1- trichlorethane peak must have a S/N ratio of not less than 5.
- For the Class 2 mixture A standard solution, the resolution between the acetonitrile and methylene chloride peaks must not be less than 1.
All the requirements are respected with the HS-GC-FID system used.
Figure 1 illustrate the analysis of Class 1, Class 2 Mixture A, and Class 2 Mixture B residual solvent mixes by Procedure A.
Procedure B
The same standard and system suitability preparations are used in Procedures A and B.
The system suitability requirements differ for Procedure B:
- For the Class 1 system suitability solution, each peak must have a S/N ratio not less than three.
- For the Class 1 standard solution, the benzene peak must have a S/N ratio of not less than five.
- For the Class 2 mixture A standard, the resolution between the acetonitrile and cis-dichloroethene peaks must not be less than one.
Again, the system suitability requirements were easily met with the used HS-GC-FID system.
Figure 2 illustrate the analysis of Class 1, Class 2 Mixture A, and Class 2 Mixture B residual solvent mixes by Procedure B.
In conclusion all products and instruments used in this technical note have been shown to reliably provide resolution and signal-to-noise ratio, as specified by USP 467.
In particular these results demonstrate that the HT200H Headspace autosampler meets the required suitability criteria for the method, and so can be used for the analysis of residual solvent in drugs.
Instrument settings